South Australian Medical Heritage Society Inc
Website for the Virtual Museum
Home
Coming meetings
Past meetings
About the Society
Main Galleries
Medicine
Surgery
Anaesthesia
X-rays
Hospitals,other organisations
Individuals of note
Small Galleries
Ethnic medicine
- Aboriginal
- Chinese
- Mediterran
Development of Interventional Cardiology
ACKNOWLEDGEMENTS
We are most grateful to Dr Leo Mahar for allowing us to publish his "Development of Interventional Cardiology" talk, based on his presentation as the William Wyatt Oration (2013), an annual address to the RAH staff, and open to the general public; and to the SAMHS in August, 2016.
Introduction
Cardiology has a long and strong history at the Royal Adelaide Hospital. Eric Gartrell brought back the first ECG machine and started the Cardiac Clinic. The Cardiac Ward was developed in the 1950's. Right heart catheterisation began with Hugh Gilmore in 1954. He was joined by John Waddy and later Elton Goldblatt who did the paediatric work. Left heart catheterisation (using arterial cut down) was introduced by Peter Hetzel in 1959, and coronary angiography was brought to the hospital by Robert Craig in 1968.
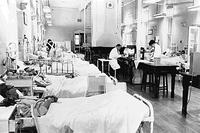
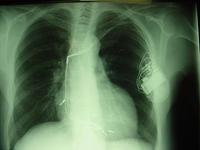
Initially, some cardiac patients were managed by Cardiologists in ward A4 along with the Cardiothoracic Surgical patients. There was no dedicated Coronary Care Unit until 1969. I can remember looking after some myocardial infarction patients in one of the long wards, as portrayed above, until 1969. In that year, a new Intensive Care and Coronary Care Unit was established in the newly opened North Wing Ward – Q4.
Intervention
Intervention is defined in the Oxford English Dictionary as "to take a course of action to change an outcome or result". Initially, Cardiologists used cardiac catheterisation to make diagnoses of anatomy and physiology, and therefore the appropriate diagnosis that was necessary for the proper surgical procedure to follow.
Rashkind Balloon Septostomy
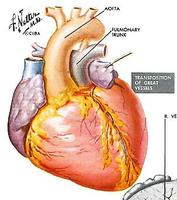
The first procedure done by Cardiologists to change physiology and anatomy was undertaken for the transposition of the great arteries. This was first done by paediatric cardiologist Rashkind in the Philadelphia Children's Hospital in 1966.
This is a diagram of the transposition of the great arteries. In this, the aorta arises from the right ventricle and the pulmonary artery from the left ventricle. When the patent ductus arteriosus closes, there are two completely separate parallel circulations not consistent with life. To enable mixing of the circulations, a balloon was pulled through the inter-atrial septum, thus mixing the venous and arterial blood to keep the child alive and allow growth. A more definitive operation could be undertaken later.
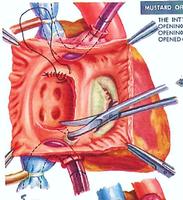
After this palliative procedure of atrial septostomy, and the child had grown and was stable, the definitive palliative procedure of excision of the inter-atrial septum and reconstruction using pericardium to create baffles to redirect the venous return was undertaken. Then the pulmonary venous return was directed through the tricuspid valve into the right ventricle, and pumped out through the aorta into the systemic circulation. The systemic venous return was directed through the mitral valve into the left ventricle, and pumped out through the pulmonary artery to be oxygenated in the lungs. This procedure could keep a child alive and be able to grow normally. I have known many people to survive into their fourth decade before the right ventricle starts to fail because of having to maintain a systemic load. Then they are referred for heart transplantation.
Dr Andreas Grüntzig
Coronary angioplasty is a very significant development in the management of angina, and now acute myocardial infarction.
Initially some arterial angioplasty was undertaken using stiff catheters, usually in the femoral artery for femoral occlusions. This work was done by Dotter in California. Andreas Grüntzig (pictured above) was the one who developed balloon catheters and appropriate guide wires to enable coronary balloon angioplasty. Andreas Grüntzig was an amazing man. He was born in the former East Germany, and along with his family, escaped into West Germany during the time of the Cold War. He migrated to Argentina where he undertook medical training and also training in radiology. He was very interested in angioplasty. He returned to Germany and started to work there, but met considerable resistance, so he transferred to Zurich. His initial attempts at angioplasty (not on live people) were unsuccessful because he was unable to get a balloon to a high enough pressure to crack the often very hard plaque. Initial balloons were silastic, the same sort of material as in a Foley (urinary) catheter. After collaboration with materials engineers, he changed to polyvinyl chloride (PVC) and was then able to get the pressure up to 8 – 10 atmospheres, which was enough to crack hard plaque (the usual pressure in a car tyre is 2 atmospheres). There are now balloons that are able to go up to 20 atmospheres which can be used after stenting.
Andreas did his first presentation of this technique in 1976, and his first patient in 1977. That patient, as far as I know, is still alive. He worked for a while in Zurich and was enticed to go to Emory University Hospital in Atlanta, Georgia, USA. He presided over the education and training of many people, developed the technique, and may well have been in line for a Nobel Prize, but he was killed while flying his plane in the mid-1980's.
He had come to Australia in 1983 (Melbourne) and 1984 (Sydney) to teach the local cardiologists how to select cases and perform the procedure. It looked like the procedure was going to be successful and spread and around the world. The first case in Australia was done at the Royal Melbourne Hospital in 1980, and we started performing the procedure at the Royal Adelaide Hospital in 1982.
Initially the balloons were very crude, with just a stiff wire at the end of a catheter and some side holes to measure pressure. The guiding catheter was very large (9F) and in itself caused some problems. Initial equipment for angioplasty cost in the order of $5,000 in today's money.
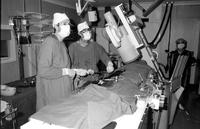
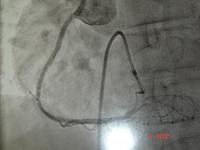
The procedure was done under sedation and local anaesthesia in the cardiac catheterisation laboratory, as pictured above.
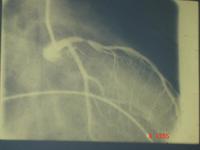
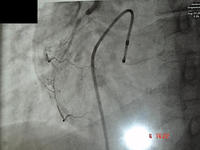
Because of the equipment, the initial lesions were quite proximal. This is a good example of the sort of lesion in the left anterior descending, which had a successful balloon angioplasty in the early 1980's.
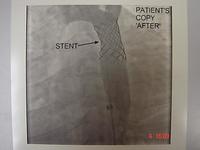
Balloon angioplasty is an uncontrolled procedure, so there was a risk of getting significant intimal tears, which can cause abrupt closures. Abrupt closures occurred in 3% - 5% of patients. We had to rush the patient back to the catheter laboratory and repeat the angioplasty, or, very occasionally, they had to go to surgery. Initially all of the patients were prepared for surgery. There was a 20% - 30% restenosis rate, which meant that the procedure had to be repeated in 3 – 6 months. A lot of devices were invented (now abandoned) to try to solve this problem. Coronary stents were introduced in 1989, initially for "bail out" of acute dissections, but they have improved enormously and now are almost routine in use in angioplasty.
Equipment improved dramatically, allowing a rapid expansion in the use of the procedures and indications, and stents are now almost routine. To prevent restenosis we now have drug eluting stents which reduces restenosis to less than 2% or 3%, which leads to a widening indication for the use of angioplasty.
There were many developments, including "over the wire" balloons, where a wire is put down to the distal part of the coronary artery, and then a balloon is slid over it. This enables a guiding track for balloon angioplasty, followed by stents, etc.
There are a number of clever devices involved, including atherectomy catheters, to carve out the atheromatous plaque which led to larger lumens, and a phase of laser catheters (now abandoned).
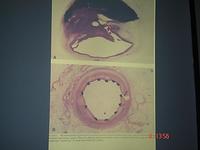
The introduction of stents has enabled the procedure to be much safer and more robust. On the top of the slide below you can see the thrombus in a dissected artery; and below, a cross-section of a stent, which shows how the artery is propped open giving a wide lumen.
As more was done, more problems were encountered. This one patient came having had multiple stents put into his right coronary following an acute dissection. He had a long narrowing. We were able to get the result below by passing a wire down, then a rotoblator catheter (like a small dental drill) was used to drill out the core, and then we gave 18 gray of intra-luminal radiotherapy to stop restenosis. These techniques are not frequently used, but they are available.
-
This is a graph of the number of coronary angioplasties done at the Royal Adelaide Hospital in the first 20 years of the procedure. As the equipment became better the indications widened, a lot more were done, and many more centres started. 1995 was the year at the Royal Adelaide Hospital when more angioplasty was done than coronary artery bypass grafting.
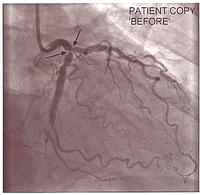
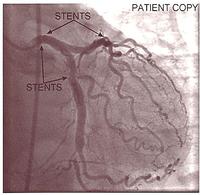
The equipment improved, and as a result of world wide clinical trials, we knew it was safe to do even a severe left main coronary artery stenosis with a dual stenting procedure. With satisfactory stenting of the left main and the proximal left anterior descending, a side hole could be made through the stent mesh into the circumflex, and further stents put in. This ended up being a very good result and kept this patient angina-free for many years.
- Stent in left main coronary artery to left anterior descending, then stent to left circumflex via side hole.
It wasn't until the 1980's that we started to understand the pathology of acute myocardial infarction. Ground-breaking work by Michael Davies and Tony Davis (pathologists in London) found that the critical pathology was a ruptured plaque. Also about that time, coronary angiography was done at the time of acute myocardial infarction, and it was found that the artery was completely occluded. As a result of this, people started to use thrombolysis, and later, balloon angioplasty, to recanalise an occluded coronary artery at the time of an acute myocardial infarction.
An acute myocardial infarction was treated by balloon angioplasty sporadically in the 1980's, during the working hours in the 1990's, and in 1997 we started a full 24/7 service with ambulance activation of the catheter laboratory so the team was ready and waiting when the patient arrived at the hospital. This is now a widespread service throughout the public hospitals and in some private hospitals. It saved some lives. It certainly saved a lot of people from getting severe myocardial infarction resulting in heart failure.
Electrophysiology
Complete heart block has a 50% annual mortality. This is when there is no activation of the ventricles from the sinus node or the atrium. Patients would often present with syncope and sudden death. Pacemakers were first introduced in 1958. They were large and unwieldy, and somewhat unreliable. They quickly developed. At the RAH, it started as a surgical procedure in 1962. Shortly afterwards it became a cardiologist procedure. Batteries improved, the leads improved, and now pacemakers are widely used and very reliable. There has been significant development over the years, from simple ventricular pacing at 70 bpm with no rate response, to now being bi-ventricular (pacing both ventricles) and associated with a defibrillator. It is amazing how this technology has developed over the last 30 years. Some pacing systems are now available without leads and are screwed into the ventricular endocardium.
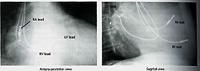
Below is an x-ray of someone who has a dual chamber pacing system associated with a defibrillator. The defibrillator coils can be seen overlying the spine, and pacing leads can be seen in the apex of the right ventricle and the right atrial appendage.
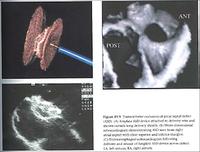
Some patients with heart failure, particularly those with a widened QRS (pulse waveform) on ECG, can benefit from pacing the right atrium, the right ventricular apex, or the outflow tract; and the left ventricle (via a coronary vein). This can considerably improve cardiac function and outlook. It is now widely practised. In these slides, pacing leads can be seen in the right ventricular apex, the right atrium lead on the left side of the spine, and on the lateral (side) view we can see a lead pacing the back wall of the left ventricle via the coronary sinus and a coronary vein.
Supra-ventricular Tachycardias
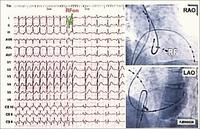
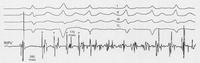
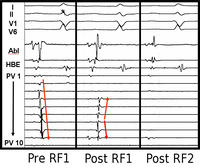
Supra-ventricular tachycardias are quite common in the community, and are often associated either with Wolff Parkinson White Syndrome (where an aberrant connection between the atrium and ventricle is present) or dual pathways in the atrio-ventricular node. For many years, the only form of treatment was anti-arrhythmic drugs. These were not always successful, and sometimes harmful. During the 1970's more accurate mappings of these arrhythmias were developed and pathways could be identified. Initially the only treatment was surgical ablation. Eventually, forms of ablative therapy via catheters were developed, either radio-frequency (heat) or cryoablation (cold). This has revolutionised a lot of people’s lives. To understand the physiology and anatomy of this condition involves sometimes 3 or 4 catheters being put into the heart, and a careful mapping of the electrical activity. With programmed stimulation, the site of the pathway could be determined by starting and stopping the tachycardia. When the site of the abnormal pathway has been determined, it could be ablated using radio-frequency. In the slide below you can see a tachycardia of 160 bpm, the pathway is identified and ablated, and the patient just returns to sinus rhythm.
Atrial Flutter
Atrial flutter is due to a right atrial re-entry electrical circuit. By ablating a narrow pathway between the coronary sinus and the tricuspid valve, this can be cured in 90% of cases with radio-frequency ablation. There is some recurrence, but this procedure can usually be repeated.
Atrial Fibrillation
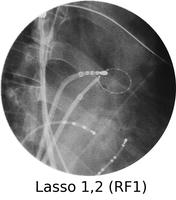
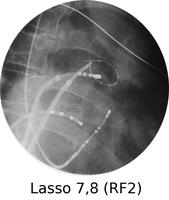
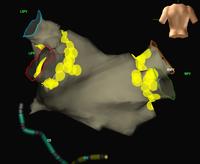
Atrial fibrillation is a very common rhythm. It is now known to originate from the left atrium and usually the pulmonary veins. The physiology is now understood much better as a result of extensive mapping, particularly of the left atrium, which requires an inter-atrial septal puncture. The atrial fibrillation electrical activity can be isolated to the pulmonary veins by creating a barrier of scar tissue around the orifice of the pulmonary veins at the left atrium.
By using x-ray guidance, including opacification of the pulmonary vein and proper placement of the catheters, it can be seen on the mapping in the electrogram (see below) that the right inferior pulmonary vein is the source of the atrial fibrillation. It is a somewhat long procedure using sophisticated imaging and electrical mapping. With appropriate RF ablation, the irregular activity can be confined to the pulmonary vein, allowing the patient to return to sinus rhythm. Each of the yellow spots show where radio-frequency ablation has been undertaken around the mouth of the pulmonary veins. The grey blob in the centre is the left atrium.
Ventricular Fibrillation
Ventricular fibrillation is of course a fatal condition unless you are very close to a defibrillator. It was the only thing that could be done initially in coronary care (defibrillate someone who went into ventricular fibrillation). A lot of ventricular fibrillation does not occur in the Coronary Care Unit, so automatic implantable defibrillator systems were developed in the 1980's. They have now become much smaller and widely used. The first case at the Royal Adelaide Hospital was done in 1994, and was a surgical procedure. It is now almost an outpatient procedure. Occasionally a patient will develop “storms” of ventricular tachycardia which require ablation. By mapping ventricular electrical activity, the site of the ventricular ectopic activity can be determined and ablated, preventing some ventricular fibrillation episodes.
Structural Heart Disease
Since the start of interventional cardiology with the Rashkind procedure, balloons have been used in other areas as well. Balloon valvotomy can be undertaken in tricuspid stenosis (a rare condition), pulmonary stenosis, or the mitral or aortic valves. It can also be used for co-arctation of the aorta.
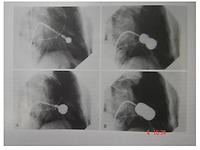
This is an example of mitral balloon valvotomy with an Inoue balloon. The guiding catheter is put through the inter-atrial septum and a balloon is advanced through the stenotic mitral valve and then slowly dilated, as seen here, until a good result is obtained. This has been a very good result. It is now a widely used procedure, particularly in Asian countries.
The patient had recurrent co-arctation of his aorta. Here the balloon and stent has produced a very satisfactory result.
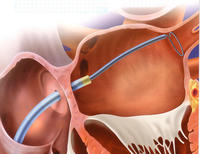
Other structural heart disease can be effectively managed with percutaneous procedures. An atrial septal defect (a hole in the septum) can be closed with a clam-like device. Patent foramen ovale can be closed again with a clam-like device, and the patent ductus arteriosis can also be closed.
A closure device is made of nitinol, which can be deformed but returns to it's original shape. The atrial septal defect can be closed by putting this device in the atrium, through the atrial septal defect, letting one side of the clam go in the left atrial side, withdrawing the device a little bit, and then letting the other side go on the right atrial side. Very quickly, the atrial septal defect will be closed. The device is seen in the upper left hand of the picture above. An echocardiogram is seen in the bottom left-hand corner with the atrial septal defect now closed.
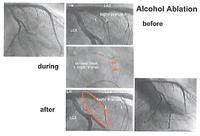
Hypertrophic cardiomyopathy is a genetic disorder of cardiac muscle cells which sometimes leads to a very bulky inter-ventricular septum. The patient symptoms of chest pain and breathlessness can be much improved by de-bulking this. This used to be done surgically. It can now be done by infarcting the area with pure alcohol put down the septal perforators. This is seen here in a series of slides.
A very high intra-ventricular pressure gradient before ablation can be seen in the image below, and after the ablation, that intra-ventricular gradient has been abolished. This improves symptoms and (hopefully) longevity.
Percutaneous aortic valve replacement is quite commonly done for severe aortic stenosis in the elderly where surgical valve replacement is a high risk procedure.
Occasionally, torrential mitral regurgitation can be improved by clipping together the leaflets of the mitral valve.
This slide shows that an aortic valve has been inserted percutaneously using the femoral artery (or occasionally axillary artery). This technique has now improved considerably, and in world leading centres it is almost a day procedure. We have an increasing population of elderly people, many of whom will develop severe aortic stenosis. This has been a very significant advance.
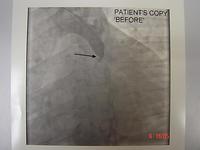
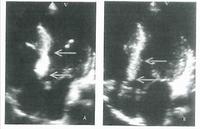
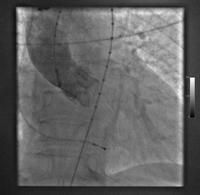
This slide shows someone who has both aortic and mitral valves in place. The patient has developed a small leak (indicated by the white arrow) with a pseudo-aneurysm, which was successfully managed using a closure device. The shadow near the top centre is a trans-oesophageal echocardiogram probe.
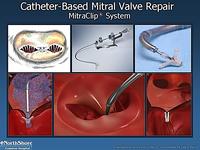
The picture below shows a catheter based mitral valve repair. This was initially done by surgeons by stitching together the leaflets of the mitral valve when there was a high risk for a mechanical mitral valve replacement. By stitching these together (the original Alferi stitch), torrential mitral regurgitation was considerably reduced. This can now be done percutaneously using a clip as demonstrated in a series of diagrams below.
This slide is a composite of things showing how cardiology has improved enormously. This has been the culmination of collaboration, innovation and dedication of many cardiologists, cardiac surgeons, nurses and technical staff, the manufacturers of the equipment, and the pharmaceutical industry.
 Rates of death due to heart disease.
Rates of death due to heart disease.
Source: NEJM.The rate of early deaths from cardiovascular disease has decreased dramatically from the highs in the 1950's, down to about one third of what it once was in the current era.
I have been involved with this over 40 years and I have seen a lot of this happen and helped to introduce some of it. It has been a most fascinating and rewarding career.
Dr Leo Mahar
-o0o-