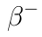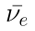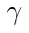
South Australian Medical Heritage Society Inc
Website for the Virtual Museum
Home
Coming meetings
Past meetings
About the Society
Main Galleries
Medicine
Surgery
Anaesthesia
X-rays
Hospitals,other organisations
Individuals of note
Small Galleries
Ethnic medicine
- Aboriginal
- Chinese
- Mediterran
Nuclear Medicine In South Australia 1960-1975 And Now
ACKNOWLEDGEMENTS: We are most grateful to Professor Barry Chatterton, FRCP DDU Head of Nuclear Medicine the Royal Adelaide Hospital (RAH), who provided most of the photographs, valuable corrections and the links; Drs. Millicent Marion (Retired), RAH, WACH (Women's and Children's Hospital) and Flinders Medical Centre, and Frans De Zwart, from The Queen Elizabeth Hospital for their kind help and advice. We are also grateful to Professor Donald Simpson for helpful suggestions.
After Roentgen discovered X-rays in 1896 it became possible to visualise lungs, bones and soft tissues. Specific organ imaging was later improved in the 1930's by using contrast media, such as the iodine-containing Hypaque (eg kidneys), barium sulphate (barium meal and enema) and thorium dioxide (Thorotrast, used to study arteries (carotid angiography)). These radiological procedures were widely used in the 1930's. Please see Wikipedia's entry on Contrast Dye for more details.
The next advance occurred mainly after WW2 and involved the use of isotopes. It resulted in the development of Nuclear Medicine.
Nuclear Medicine is closely related to the discovery of "mysterious rays" by Henri Becquerel
in 1896. Marie Curie (nee Sklodowska) a year later had coined the term "radioactivity". It became evident
that many elements existed in different forms, known as isotopes. Some of these isotopes are unstable,
undergoing radioactive decay which can be measured.
Unlike X-rays, which are generated outside the patient, pass through and are recorded on a photographic
plate; radioactive isotopes were used to label chemical agents, the combination known as a
radio-pharmaceutical. These could be injected,
ingested, or inhaled and accumulate with varying specificity in the organs such as liver, brain, thyroid,
lungs and bone. Their activity was detected by specific counters sited outside the body, or recorded on a film
forming images of normal and abnormal organs.
The invention of the Cyclotron by E. O. Lawrence at Berkeley as early as 1930's made it
possible to produce many types of isotopes. The number available became much more numerous after the
Manhattan project gave rise to the atomic bomb and many nuclear reactors were constructed.
With the increased number of commercial or semi-commercial sources (e.g. Amersham (UK), Oak Ridge (US)
and Lucas Heights (Australia)), radiopharmaceuticals became more widely available.
Iodine-131 (131I) was one of the earliest isotopes used to image the thyroid gland. It was
also used for treating the hyperactive thyroid disease.
Technetium 99m was discovered by Segre and Seaborg
in 1938. Twenty years later it became a popular imaging isotope in the 1960's. It has a half life of 6 hours,
and could be eluted from 99 Molybdate as Tc99m-pertechnetate. The apparatus was usually referred to as the
"technetium cow". Because of its predominately gamma decay, short biological retention, and easily detected
gamma emissions (approximately 140 keV), Tc-99m became a favourite scanning isotope.
Imaging was originally performed by using a linear scanner, which was passed across an elected site such as the neck, skull, or the abdomen, in a series of horizontal lines and the radioactivity (count value) was recorded, thus producing a crude image of the organ. A variety of tracers were used, eg Arsenic-74 (74As) was used in the very early brain scans, but not in South Australia.
In 1957, Hal Anger and B. Cassen developed the scintillation camera (Gamma camera), which was a major breakthrough as the whole organ could be imaged at once. The photographs were recorded on a polaroid film, 35 mm. film, or x-ray film. With advances in computing, they were acquired digitally. The images were adequate, but the resolution was limited to little more than a centimetre or so.
Nuclear medicine was one of the early advances in organ imaging. It was used extensively to
show any abnormalities in many organs such as the brain, liver, thyroid, and lungs in the 1960's and 1970's.
Although the number of brain and liver scans performed now is low, there has been a large increase in bone
and heart scans as well as "malignancy" positron emission tomography (PET) scans. Compared to 1972,
in 2013 the number of scans in South Australia has increased 5 fold, with some of the early indications
such as placental scanning and liver scans for anatomy being obsolete, but newer indications and
radiopharmaceuticals taking their place.
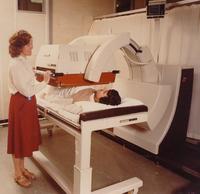
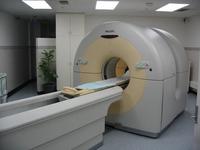
Developments in other modalities followed, and computerised tomography (CT) was introduced into
SA in 1973, and magnetic resonance imaging (MRI) in 1986. These machines are very widely used. They demonstrate
anatomy better with superior resolution.
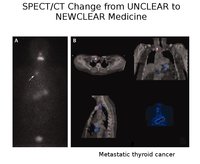
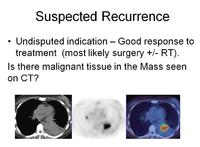
Nuclear medicine remains valuable with scans providing a functional image indicating early changes in metabolic activity before changes in size in cancer. If coupled with a CT they provide valuable information about likely prognosis and can suggest treatment modes.
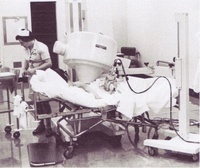
The first gamma camera in South Australia was purchased for the Royal Adelaide Hospital by
the then Reader in Medicine, Dr. Harry Lander, in 1967.
In the late 60's and 70's, Dr. Peter Ronai and Dr. Millicent Marion, both from Sydney, joined the
Nuclear Medicine Department at the RAH, and Dr. Frans De Zwart was appointed as the Director of
Nuclear Medicine at the Queen Elizabeth Hospital. Drs. Joe Savage and John Arnold headed the Departments
at the (then) Adelaide Children's Hospital and the Flinders Medical Centre.
Drs. Ronai and Marion introduced into the state bone and kidney scanning, and the first practical
technetium biliary agent was developed in Adelaide.
Fluorine-18 (18F), a high energy positron emitter, was first used at the RAH in the late 1960's
with bone scanning the first clinical use of the "PET" scan, however the supply chain was difficult,
and the electronics and lack of computing power made the technique unwieldy.
Now the commonly used molecule is 18FDG (fluorodeoxy-glucose). When coupled with a CT or MRI
image it gives a compound image of exceptional anatomical quality.
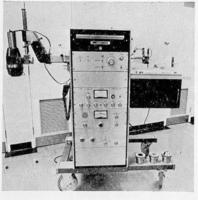
The following photographs provided by Drs. Chatterton, Marion and De Zwart show some of the early and more recent machines and their pictures.
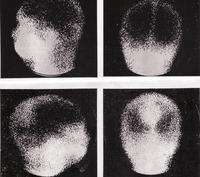
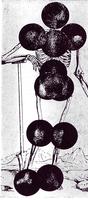
SOME OF THE PHOTOGRAPHS RECORDED BY THE GAMMA CAMERA AT THE RAH IN 1968.
THE ABOVE PHOTOGRAPHS REFLECT ONE OF THE SIMPLE ADVANCES IN TECHNOLOGY. BOTH CAMERAS CAN BE ROTATED AND THUS REMOVE THE NEED FOR THE PATIENT ROTATION.
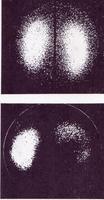
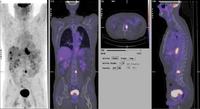
RECENT IMAGING PHOTOGRAPHS FROM THE RAH COURTESY OF PROF CHATTERTON.
Appendix 1
Dr Frans de Zwart has kindly written to us about his period at The Queen Elizabeth Hospital in the Department of Nuclear Medicine.
Dr de Zwart was responsible for several innovations in his field. The original equipment of his department consisted of a Toshiba large field gamma camera, a scintillation probe, and a Nikon SLR camera. The latter was chosen in preference to the commonly used Polaroid camera because of the more rapid acquisition of pictures, and the resolution was adequate for reporting. The final photographs were enlarged for use in the case notes.
Dr de Zwart's staff included Dr L. Barnden. a physicist who was responsible for many of the calculations and adjustments which were required; Mr Colin Hentschke, a photographer who recorded and enlarged the 35 mm films; a dedicated nurse, and a secretary.
The renal transplantation service provided patients for studies of renal perfusion, function, and morphology in renal transplant patients.
Of the numerous (>900) studies, it was possible to first detect rejection or vascular problems using the gamma camera in about 70% of the cases.
Another activity was the introduction of the "first pass" cardiac studies and a cardiac exercise apparatus which was funded by local commercial firms.
Finally, dedicated co-axial cables were installed to the Renal Unit (transplant information), and the Intensive Care Unit. A simple 30 second brain flow study was sufficient to confirm brain death, and thus suggest possible renal donors.
Later development of CT, MRI, and SPECT technologies changed the imaging opportunities, but nuclear medicine publications still report on the monitoring of transplant patients in 2012.
A local review of the Renal Transplant Scintigraphy (parts 1 and 2) was reported in the ANZSNMT (Australian and New Zealand Society of Nuclear Medicine Transactions) in 2004 in a Continuous Professional Development Article by Dr Ghee Chew, FRACP, a Renal and Transplantation physician at The Royal Adelaide Hospital.
Appendix 2
The name 'technetium' is derived from the Greek word 'technetos', which means 'artificial'. This name was chosen because technetium was the first predominantly artificial element, with only tiny amounts naturally generated. All of the isotopes of technetium are unstable, with half-lives ranging from less than 110 ns (85Tc) to 4.6x106 years (98Tc).
The "m" in 99mTc indicates that the nucleus is in an excited state (a metastable nuclear isomer) after the decay of its parent, and will eventually decay to its ground state, either by releasing a gamma ray, or via internal electron conversion.
Due to the short half-life of 99mTc it can't be transported far, so a safe and easy way to generate it was needed. The first 99mTc generator was developed at the Brookhaven National Laboratory in 1958 by Walter Tucker and Margaret Greene.
The parent element of 99mTc, 99Mo, has a half-life of 66 hours (2 3/4 days), and is therefore able to be transported much further, but needs to be replaced weekly (approx 12% remaining after 3 half-lives, or 8 days).
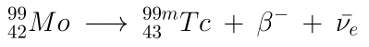
Where:
: beta particle (electron) : electron antineutrino
Approximately 1 in 8 99Mo nuclei may decay into 99Tc directly.
Most generators consist of a radiation shielding container, and Mo-99 molybdate ( 99MoO42- ) adsorbed onto an acidic alumina (Al2O3, approx pH 4.5 ) substrate. The molybdate decays into pertechnetate ( 99mTcO4- ). The pertechnetate is then eluted, or washed, from the generator with a normal saline solution.
This sodium pertechnetate solution can either be used by itself, or added to a organ-specific pharmaceutical for a targeted scan. For example, by itself, the sodium pertechnetate behaves similarly to iodine, concentrating in the thyroid, salivary glands, brain, blood pools, urinary bladder, and the stomach about 30 minutes after being administered; Tc labelled sulphur colloids accumulate in the liver, spleen, blood pool, and bone marrow; and Tc labelled macroaggregated albumin for targeting the lungs.
Because the half-life of 99Tc is much greater than that of 99mTc, the longer a generator is left unwashed, the greater the percentage of medically useless 99Tc in the eluent becomes.
In order to get the greatest ratio of 99mTc to 99Tc, generators may be milked approximately every 6 hours. The ratio starts to approach equilibrium after 4 to 5 elutions.
99mTc has a half-life of 6 hours, and decays to 99Tc. The most common mode of decay ( ~88% ) is by releasing a gamma ray. Approximately 98.6% of these rays are 140.5 keV, with the remaining 1.4% being 142.6 keV. The other ~12% of the time, 99mTc decays via internal conversion; this is when the nucleus interacts electromagnetically with one of the electrons in the inner electron shells, causing the electron to be ejected. This ejected electron has an energy of ~140 keV, and ionizes tissue in same way as beta particles do. These ~140 keV (8.8 pm) gamma rays are easily detected by conventional X-ray detectors.
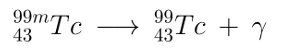
Where:
: gamma photon
The resultant 99Tc has a half-life of 211,100 years, which eventually beta decays ( 249 keV ) to the stable ruthenium-99.
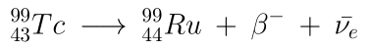
-o0o-